If it's not clean, it's not sterile: What does clinically clean mean?
Even with strong guidance from the CDC and FDA, not all medical detergents deliver clean efficiently. This can result in medical instruments with soils reaching the operating room. Is this due to improper detergent testing, poor cleaning protocols or something entirely different?
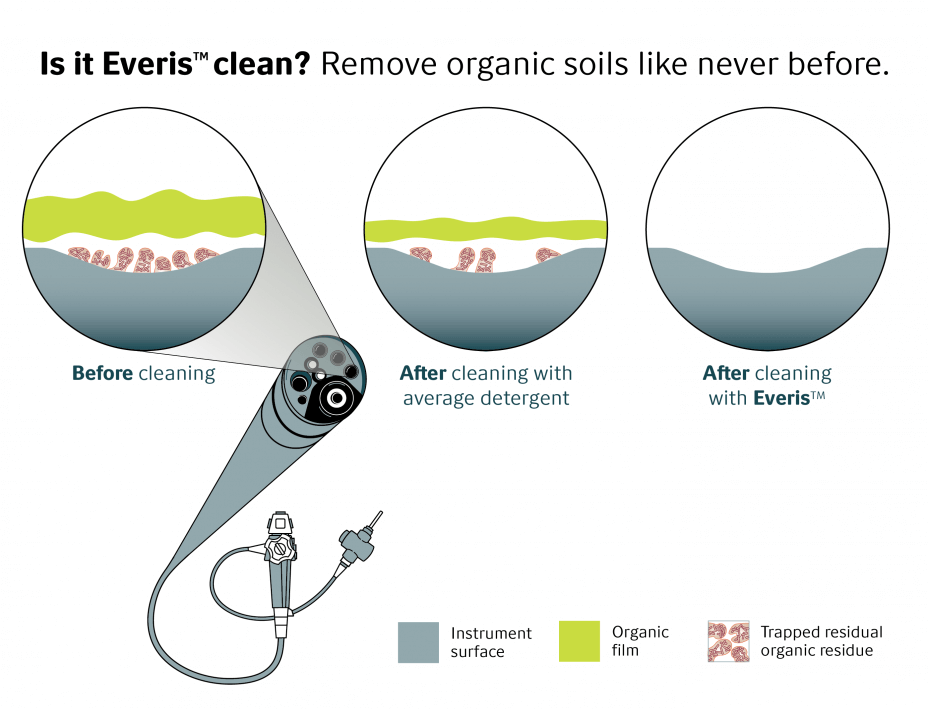
FDA and CDC guidelines, among others, note that cleaning is the first and most critical step in medical device reprocessing. If a medical instrument is not completely clean, it may contain residual soil that traps and protects bacteria, preventing subsequent disinfection and/or sterilization steps from effectively killing them. No one wants visible or invisible soils from a previous patient on the instrument they are using. When present, it is reflective of poor cleaning standards, defective cleaning equipment or inadequate medical detergents.
Medical instruments are not cleaned during the disinfection and/or sterilization steps, so the cleaning that happens during the cleaning process must be effective, thorough and comprehensive. Inorganic and organic materials that remain on the surfaces of instruments not only interfere with the effectiveness of the subsequent disinfection, and/or sterilization processes, but may also leave the instrument contaminated.
The 2008 CDC guidelines define cleaning as:
- Removal, usually with detergent and water or enzyme cleaner and water, of adherent visible soil, blood, protein substances, microorganisms and other debris from the surfaces, crevices, serrations, joints, and lumens of instruments, devices, and equipment by a manual or mechanical process that prepares the items for safe handling and/or further decontamination. (Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 (cdc.gov))
And the 2015 FDA guidelines define cleaning as:
- Physical removal of soil and contaminants from an item to the extent necessary for further processing or for the intended use. (FDA)
With the FDA and CDC's emphasis on the importance of cleaning prior to disinfection and/or sterilization, why are soiled devices reaching the operating room? Detergent manufacturers do not have access to clinically soiled instruments, and in most cases test their detergents on artificial soils to gauge efficacy. Artificial soils may not adequately represent the challenges posed by clinical soils resulting in the instruments passing the cleaning and microbiocidal processes with soils still attached.
A study performed with 10 commercially available detergents demonstrated an average clinical soil removal of 51%. The best performing detergent removed 75% of the clinical soil and the worst one only removed 20%.
A more recent study has demonstrated that using the right Novozymes Everis™ enzyme product, with the proper dose and under the right conditions, improves cleaning outcomes on clinically soiled medical devices. Detergents optimized with Novozymes Everis™ can keep patients safer by effectively eliminating organic residues that other detergents cannot.